Use the density of water, the molar mass of water, and Avogadro's number to calculate the number of molecules of water. We have 500mL of water. Use the density to convert this to grams; then use the molar mass of water to convert this to moles. There are two moles of hydrogen atoms per one mole of water. Finally, multiply by Avogadro's number.
- Calculating Moles With Avogadro's Number Calculator
- How To Calculate The Number Of Moles Using Avogadro's
- Avogadro's Number And Mole
Moles and Avogadro’s number are big! And they come across as overwhelming. But YOU can master them! Hidetsugu ueno.
This video covers the definition of Moles, with an illustration showing the logic of moles in general chemistry. You’ll also see how to easily and quickly convert from and to them on the MCAT.
You’ll see conversions including atoms, AMU, grams, and moles. Let’s knock this out on your MCAT Content Category List!
- A mole of a substance contains N a = 6.022×1023mol−1 N a = 6.022 × 10 23 m o l − 1 number of particles of that substance, where, N a N a is known as the Avogadro's Number. If N N is the total.
- The number 6.022 X 10 23 was given the name Avogadro’s Number by Jean Perrin in 1901. In 1902 Ostwald proposed the term “ mole ” as another way to express Avogadro’s Number. So a mole of carbon ( C ) would have a mass of 12.0107 g and contain 6.022 X 10 23 atoms of carbon.
- . Generally, we round Avogadro’s number to 6.022 × 1023. Thus, just as one dozen oranges contains 12 oranges, 1 mole of hydrogen atoms contains 6.022 × 1023 H atoms. The following Figure shows samples containing 1 mole each of several common elements. (One mole each of several common elements.
- Mole Calculations I. We apply the unit factor 1 mol K/6.02. Atoms K to cancel atoms, which appears in the denominator. The answer is rounded to three digits because the given value and unit factor each have three significant digits. Calculate the number of moles of potassium iodide in 5.34 ×1025 formula units of KI.
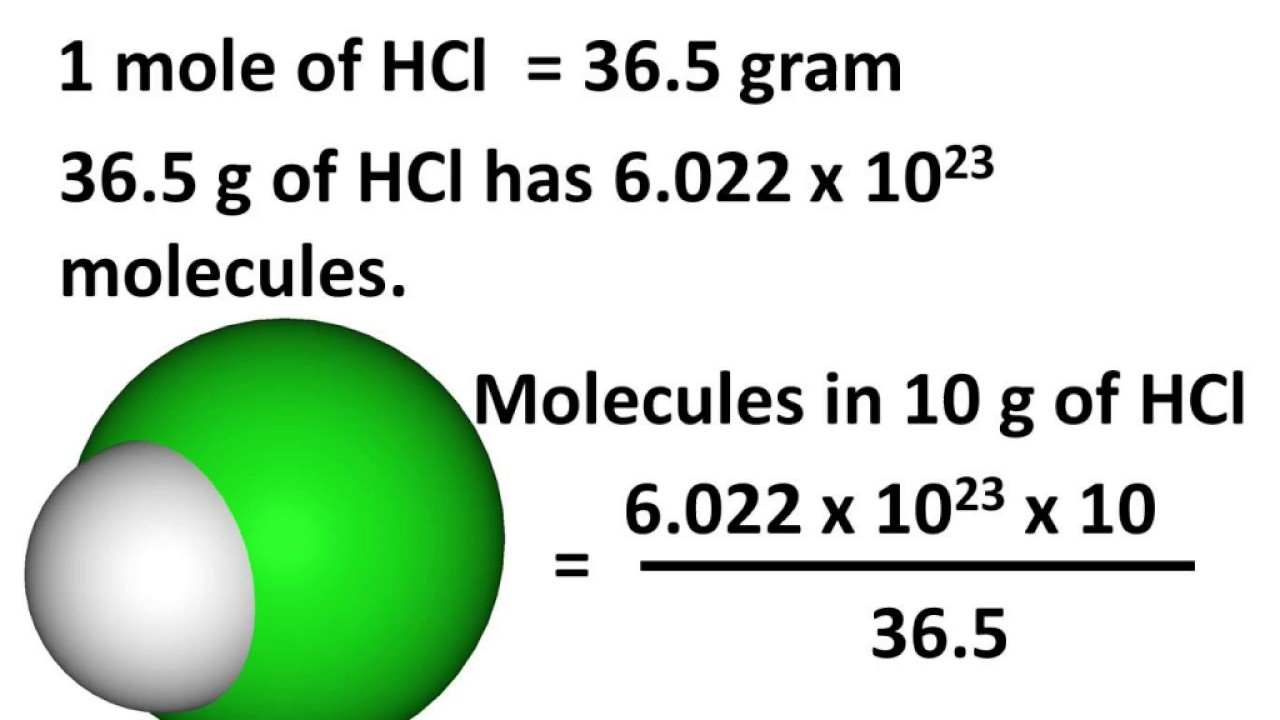


Calculating Moles With Avogadro's Number Calculator
(Watch on YouTube: Mole and Avogadro’s #. Click CC for transcription.)
How To Calculate The Number Of Moles Using Avogadro's
Links & Resources Mentioned In This Video:
<– Watch Previous Video: Percent Mass by Composition
–> Watch Next Video: Density
This is Video 6 in the Stoichiometry & Reactions Video Series. Click HERE for the entire series.
Avogadro's Number And Mole
Ready to test your knowledge? Try the MCAT Reactions and Stoichiometry practice quiz!
